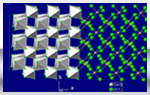
 Calcium Chloride
Calcium Chloride
| Calcium Chloride |
|
CaCl2 CaCl2 2H2O CaCl2 LIQUID |
| Purity |
| Anhydrous: 93-98% Hydrous: 73% Liquid: 25-35% |
| Process |
| Calcium chloride is a white crystalline solid. It is very soluble in water. Extremely hygroscopic and liberates large amount of heat during water absorption. |
| Chemical Reaction |
|
Calcium Chloride is prepared by the reaction Calcium Carbonate with Hydrochloric Acid. |
| Raw Materials |
|
| Application |
|